Procedure
Botox
The FDA has approved botulinum toxin type A (Botox) for treatment of chronic migraine headaches in adults. Injections are made in muscles of the face, head and neck. When this is effective, the treatment typically needs to be repeated every 12-15 weeks. Insurance will pay for this if you have failed medical treatment and have 15 headache days per month, that last at least 4 hours, for at least 3 months. In spite of recommended standard injection sites, Botox injection are somewhat an art and therefore there can be varying results depending on the experience and skill of the injector. If done properly, there are minimal risks. There is not as much data to support the use of Botox in cluster, tension or chronic daily headaches.
Nerve Block
A nerve block is an injection near a nerve. It involves use of a small needle, and medication to decrease or block the passage of nerve impulses. Nerve blocks are a unique treatment for controlling headaches. Most nerve blocks for headaches are done in the back of the head over the occipital bone and nerve and therefore are called an Occipital Nerve Block (ONB). However, any nerve on the scalp can be injected. Predicting who will respond to a nerve block is difficult. Patients with occipital neuralgia (pain from the occipital nerve) that may be causing or making worse a headache syndrome (most often migraines or cervicogenic headaches but sometimes even cluster headaches) may receive relief not only from the occipital nerve pain but from the headache syndrome as well. These blocks will last the longest in this type of patient but unfortunately, can last as little as only one day. Medications injected include a local anesthetic and a steroid or just the steroid or just anesthetic alone. Nerve blocks are generally benign, but there can be side effects. Some insurance companies will not pay for nerve blocks.
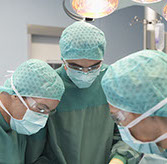
Nerve Decompression Surgery
This procedure most often involves an incision at the back of the head to remove tissue that may irritate the occipital nerve(s). However, in the last 3-4 years several Plastic and Reconstructive Surgery Programs offer a procedure to treat migraine headaches by surgically treating portions of the trigeminal nerve, a large nerve branch in the face and neck that can sometimes trigger migraines. Depending on the type of migraine, a minimally invasive technique is done to remove the portion of the nerve that is irritated or the muscle around it. Another approach targets other nerves or muscles in the face or neck. Also, reducing nasal trigger points through septoplasty, a surgical procedure for repairing deviated septum, may be done at the same time. This is a 2-3 hour operation and has risks that are real! This procedure is primarily to treat migraines, is not FDA approved and is difficult to get insurance to pay for it.
Nerve Stimulation (Implanted)
Electrodes are implanted at the base of the skull, near the occipital nerve. These are then connected to a power source that sends electrical impulses to the occipital nerve. The power source is also implanted, often under the collarbone, with the wires running under the skin to the electrodes that are sewn over the occipital nerves. Possible complications include the need for surgical revision of wire placement as they can move with physical activity. Infection also is a risk. Studies on occipital nerve stimulation so far have included only a small number of participants. Cluster headaches are the most common patients helped with this procedure with far fewer being done for migraines — and long-term results are not yet available. This procedure is not FDA approved and it may be tough to get insurance companies to pay for it.
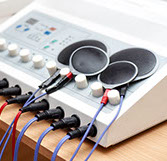
Nerve Stimulation (External)
Recently a new nerve-stimulating device to treat headache and migraine showed some benefit in a randomized, controlled study. The external device works by sending high frequency neurostimulation to the trigeminal nerve, which is frequently involved in head pain. It is worn for as little as twenty minutes per day and may reduce headaches as much as some preventative migraine medications. It may help tension and chronic daily headaches as well as migraine. It is relatively inexpensive ($350 to $425) compared to these other procedures. And, although it is not FDA approved, there is very little if any medical risks with this battery powered device.
Deep Brain Stimulation (DBS)
A stimulator is implanted in the hypothalamus, thought to be the area of the brain associated with the timing of cluster periods. There are several small studies that show DBS may provide relief for people with severe, chronic cluster headaches. There is less clear evidence for other headache types. This surgery can cause infections, bleeds and strokes and is not FDA approved for any headaches.
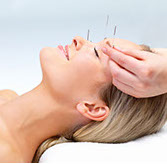
Accupuncture
The treatment consists of many thin, disposable needles inserted into several areas of your skin at defined points. Some clinical trials have found that acupuncture may be helpful for headache pain. This includes tension, migraine and chronic daily headache. However, it does not seem to provide much preventive benefit.
Site Map
Disclaimer